Raman Spectroscopy (CARS and SRS)
Raman Spectroscopy (CARS and SRS)
CARS and SRS Raman Microscopy
Raman microscopy is a technique that enables label-free chemical imaging. It is based on the Raman scattering effect of molecules that was discovered by C.V. Raman in the early 1930s. When light with a particular wavelength or photon energy is incident on a molecule, it can be inelastically scattered. This means that part of the energy is absorbed by the molecule and the scattered photon has a lower energy than the incident photon (see Figure 1). The fraction of the energy absorbed depends on the vibrational frequencies of the molecule. All molecules have specific Raman frequencies, typically given in wavenumbers, which span from 100 cm-1 to 3500 cm-1. A molecule's Raman spectrum is highly dependent on its chemical structure but mostly unaffected by the local environment. Therefore, Raman spectroscopy is not only specific but also robust to environmental variability.
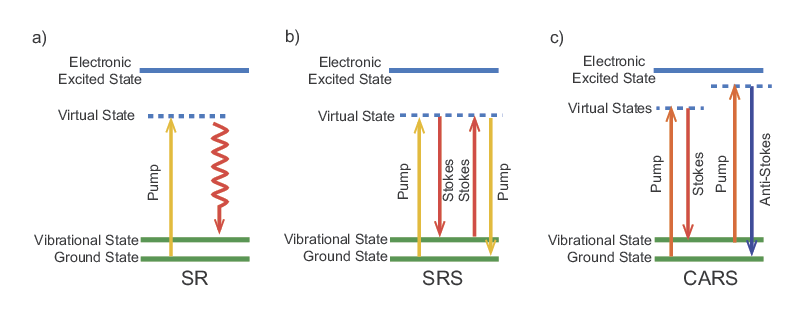
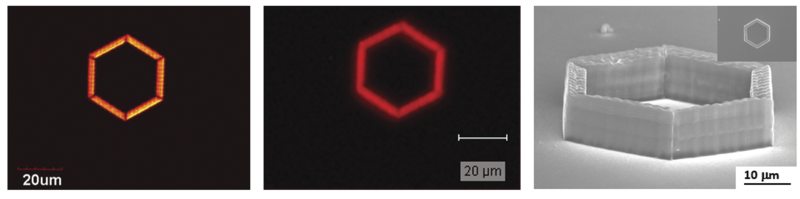
In CARS microscopy, the pump and Stokes beams are tightly focused into the sample and a CARS image is generated by scanning either the sample or the laser beams. Besides possessing the same 3D sectioning capability afforded to 2PF and 3PF microscopy, CARS microscopy offers several advantages. CARS microscopy permits non-destructive molecular imaging without any labeling. This advantage is important for imaging small molecules such as lipids where labeling may significantly affect their molecular properties. The coherent amplification from the molecular oscillators results in a highly directional output, which greatly facilitates signal collection. A comparison between images of a polymeric structure taken by CARS and SR microscopy is shown in Figure 2. The CARS image possesses higher fidelity despite an acquisition time (under comparable laser power) two orders-of-magnitude shorter than for the SR image. Finally, CARS signals appear at a wavelength that is shorter than the excitation wavelengths; therefore, spectrally-separating it from the one-photon fluorescence background is straightforward.
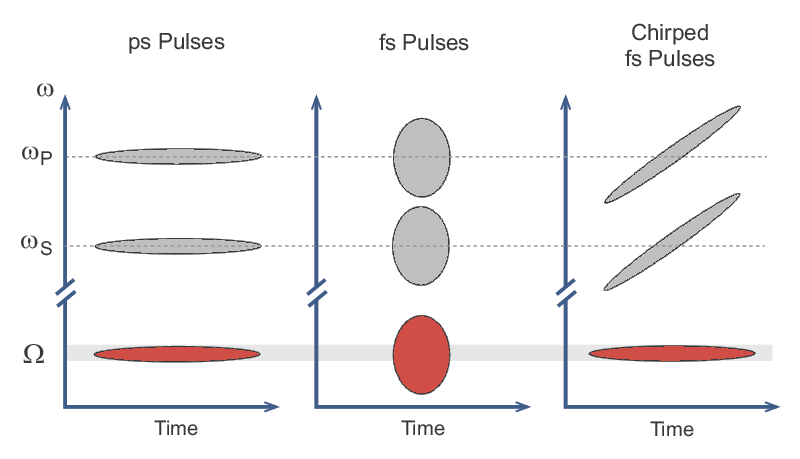
A clever solution called spectral focusing allows the full bandwidth of a fs pulse to be used efficiently for these nonlinear microscopy techniques. In this method, both the pump (ωP) and Stokes (ωS) pulses are linearly chirped as shown in Figure 3. Optical pulses where the frequency increases or decreases within the temporal pulse are referred to as chirped, in analogy with sound pulses. Therefore, in the plots shown in Figure 3, a horizontal line represents a pulse that is not chirped and the vertical width of the line represents the bandwidth of the pulse. Conversely, a tilted line indicates a linearly chirped pulse. When both the pump and the Stokes pulses are linearly chirped by the same amount, the difference between the two frequencies (Ω = ωP- ωS) can be quite narrow. Thus, two broadband fs pulses can still be used to efficiently excite a narrowband Raman line. Tuning of the frequency difference between the pulses is also possible using this technique. This is accomplished not by tuning the central wavelength of the laser but rather by changing the relative timing between the two chirped pulses. Spectral focusing allows fs lasers to be used for all nonlinear microscopy techniques including CARS and SRS.
In SFG microscopy, the fluorescent dye is omitted and the sum frequency signal is generated from the sample itself, similar to both SHG and THG microscopy. However, in contrast to these techniques where only a single wavelength is required, two different wavelengths are required for SFG. Consequently, this technique has similar requirements to CARS in that the pulses from the two lasers must arrive at the same location in the sample at the same time. Therefore, synchronized lasers are needed for SFG microscopy.
The MKS Spectra-Physics InSight® X3™ laser, when equipped with the fixed 1045 nm dual beam option, can fully support the diverse needs of multimodal imaging. The two synchronized output beams, one tunable and one at the fixed wavelength, enable simultaneous imaging of various fluorescent proteins (for example GFP and mCherry) and genetically encoded calcium indicators (GCaMP6 and jRGECO1a), SHG/THG imaging, and advanced imaging techniques such as CARS and SRS.




